
Optimize Commissioning and Qualification (C&Q) Processes with a Virtual Solution
In the dynamic biopharmaceutical landscape, Commissioning and Qualification (C&Q) processes are essential pillars, ensuring the production of cutting-edge therapies. Furthermore, with a focus on regulatory compliance, these processes guarantee seamless alignment of manufacturing facilities and equipment with FDA and EMA standards.
This meticulous adherence to industry regulations forms the foundation for a robust and reliable biopharmaceutical production ecosystem. Embracing the Virtual Twin Experience, advanced virtual simulations provide a comprehensive overview, allowing thorough testing and validation before real-world implementation. This innovative approach ensures efficient compliance, accelerates industrialization, and reduces risks, marking a significant leap forward in biopharmaceutical production.
C&Q activities include:
Verification of operations, maintenance and training manuals
Design reviews
Factory and site acceptance tests
Functional testing
The C&Q process ensures:
Correct installation of specified equipment
Proper functioning of equipment as designed
Successful handover to the appropriately trained user
Virtual Commissioning Process:
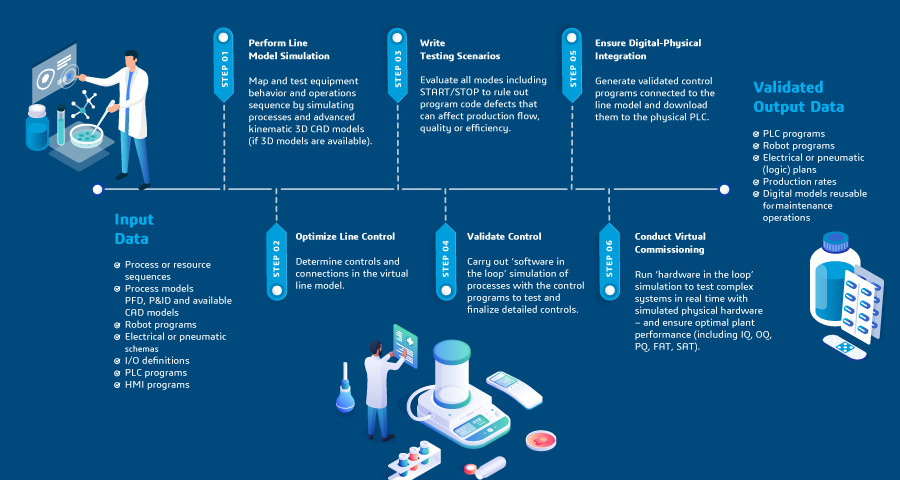
Click image to enlarge
Real-Time Plant Simulation with Virtual Commissioning
Dassault Systèmes offers expertise in building a virtual twin experience of your facility and equipment line from 3D scans or 2D drawings, ensuring rapid benefits and value. Furthermore, our outcome-based services seamlessly integrate with your projects. This comprehensive approach guarantees a streamlined process, optimizing efficiency and delivering tangible results for your biopharmaceutical commissioning endeavors.
Virtual Commissioning is Essential for Optimized Processes
Experience accelerated industrialization, reduced immobilization of assets, minimized quality risks, and increased flexibility with Dassault Systèmes’ 3DEXPERIENCE platform. Seamlessly transition into the future of biopharmaceutical commissioning for unparalleled efficiency and innovation. This integrated solution not only addresses current challenges but also establishes a foundation for continuous improvement and advancement in biopharmaceutical production processes.